

What will be the volume of this gas if the pressure becomes 3.00 atm? (1.56 L) (1.00 atm) = (3.00 atm) (x) 0.520 L 18. To what pressure must a gas be compressed in order to get into a 3.00 cubic foot tank the entire weight of a gas that occupies 400.0 cu. If a gas at 25.0 ☌ occupies 3.60 liters at a pressure of 1.00 atm, what will be its volume at a pressure of 2.50 atm? (1.00 atm) ( 3.60 liters) = (2.50 atm) (x) x = 1.44 L 16. What is the volume when the pressure is increased to 60.0 mmHg? (40.0 mmHg) (12.3 liters) = (60.0 mmHg) (x) x = 8.20 L 15. A gas occupies 12.3 liters at a pressure of 40.0 mmHg. Calculate the total volume occupied by the gas. mL flask with 46.65 kPa pressure of gas, then expanded it into the apparatus. In order to measure the volume of a piece of apparatus, a chemist filled a 750. The first bulb has a volume of 56.0 mL and contains 5.92 atm of argon, the second bulb has a volume of 250.0 mL and contains 1.28 atm of neon, and the third bulb has a volume of 37.0 mL and contains 8.50 atm of hydrogen. Three bulbs are connected by tubing, and the tubing is evacuated. When the two flasks are connected the pressure in the system drops to 2 atm. An evacuated flask A, which has a volume of 30 mL, is attached to a second flask B containing an ideal gas at a pressure of 5 atm. What pressure is required to compress 196.0 liters of air at 1.00 atmosphere into a cylinder whose volume is 26.0 liters? (1.00 atm) (196.0 L) = (x) (26.0 L) x = 7.54 atm 11.

If we have 6.00 cm3 of gas at a pressure of 10.0 N/cm2 and we increase the pressure to 20.0 N/cm2, what volume will the gas occupy? (10.0) (6.00) = (20.0) (x) x = 3.00 cm3 10. However, at standard pressure, its volume was measured to be 8.00 L. 9.48 L of a gas was at an unknown pressure. 2.00 L of a gas is at 740.0 mmHg pressure. What will be the volume of the CO2 if the pressure is increased to 795 torr? P1 = 742 torr P2 = 795 torr V1 = 500. What is the volume of the propane at standard pressure? P1 = 830. A gas tank holds 2785 L of propane, C3H8, at 830.
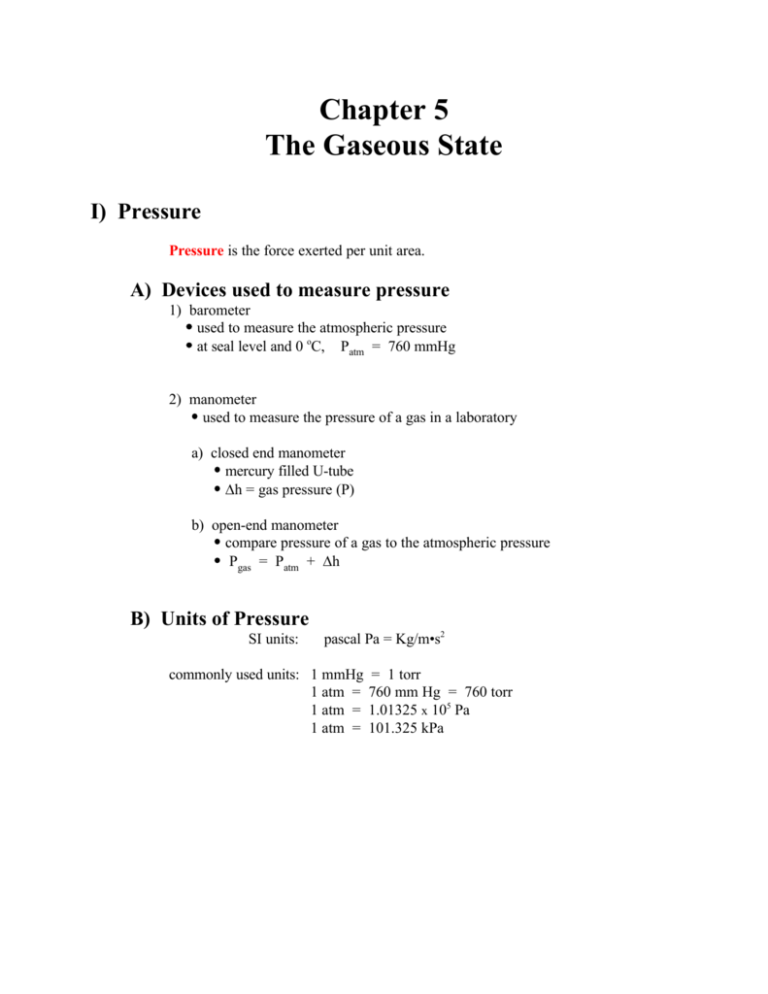
What was the initial pressure exerted on the balloon? P1 = ? P2 = 2.00 atm V1 = 7.2 L V2 = 25.1 L P1V1 = P2V2 P1 = P2V2/V1 P1 = 7.0 atm 4. The pressure is reduced to 2.00 atm and the balloon expands to occupy a volume of 25.1 L. What will be the volume of the neon when the pressure is reduced to 93.3 kPa? P1 = 101.3 kPa P2 = 93.3 kPa V1 = 461 mL V2 = ? P1V1 = P2V2 V2 = P1V1/P2 V2 = 501 mL Ne 3. A sample of neon occupies a volume of 461 mL at STP. What is the volume of the container in liters? P1 = 680. The temperature remains constant at 296 K. mm Hg are placed in a container under a pressure of 1210 mm Hg. 352 mL of chlorine under a pressure of 680.


 0 kommentar(er)
0 kommentar(er)
